FDA approves first over-the-counter Narcan for opioid overdose treatment
TAMPA, Fla. - The US Food and Drug Administration approved Narcan nasal spray for over-the-counter use on Wednesday.
It’s the first naloxone product approved for use without a prescription. The lifesaving medication reverses an opioid overdose, and the FDA says the approval paves the way for it to be sold directly to consumers in drug stores, convenience stores, grocery stores, gas stations as well as online.
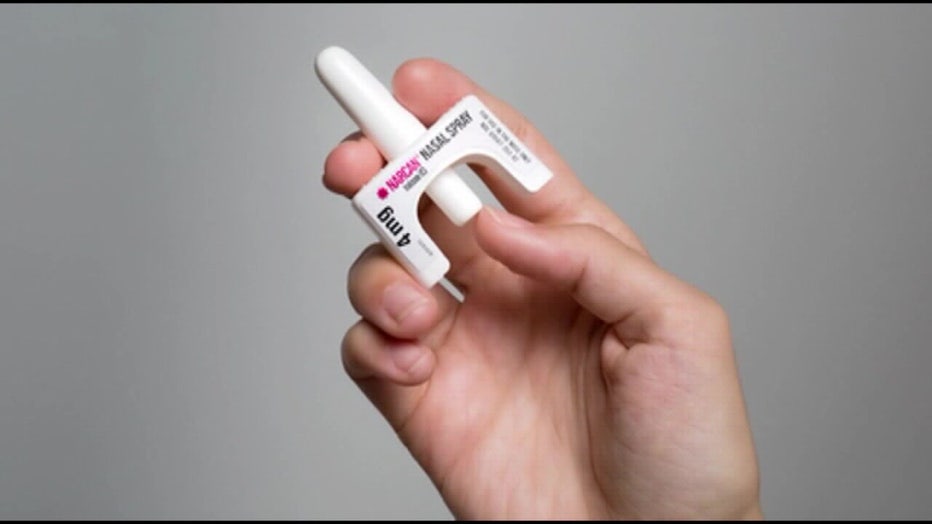
"Today’s approval of OTC naloxone nasal spray will help improve access to naloxone, increase the number of locations where it’s available and help reduce opioid overdose deaths throughout the country," FDA Commissioner Robert M. Califf, M.D., said. "We encourage the manufacturer to make accessibility to the product a priority by making it available as soon as possible and at an affordable price."
READ: Florida attorney general warns of 'zombie drug' after hundreds of overdose deaths
Local addiction treatment centers say that increased access will be key to reducing overdose deaths.
"It’s so important for people to have access. And I think with our population having access over the counter, it's going to be even more beneficial because there's no stigma attached to asking," said Robin Piper, the CEO of Turning Point of Tampa.
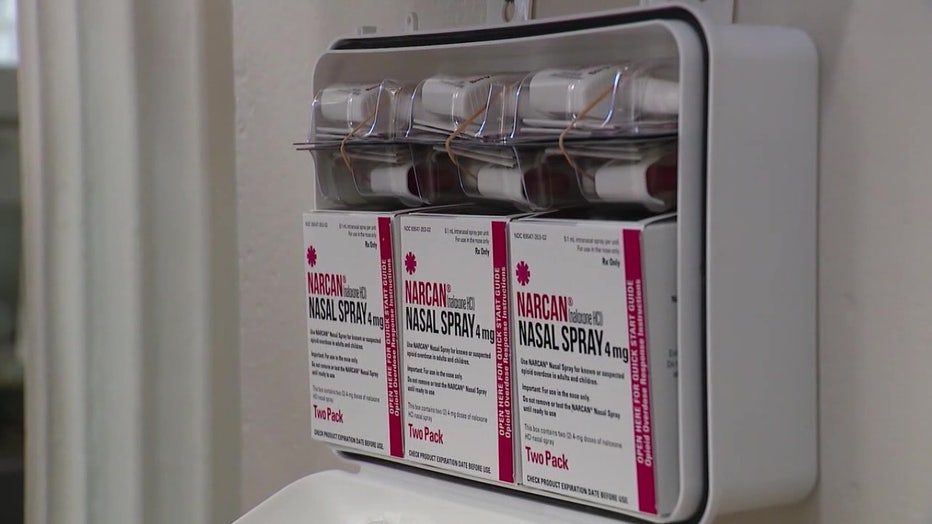
The FDA says the price of the OTC version is determined by the manufacturer. Doctors say they hope cost isn’t something that becomes a barrier to access.
"I am also very sensitive to ensure that people that may not have the resources to buy it are not penalized by the fact that they now have to pay out of pocket," Dr. Nora Volkow, the National Institute on Drug Abuse director, said.
It’s expected the over-the-counter version will be available by late summer.

